Info Overview
Prevention
There are some excellent information resources on Hereditory Hemochromatosis(HH). Each country has a charitable or semi-charitable organisation which provides very useful help and advice for people diagnosed and suffering from Hereditory Hemochromatosis. The problem we have concerning these information resources is they provide no positive help for prevention of Iron Overload and little to no information on how genetics can be used to determine someone's risks. The reason for these ommisions is historical as 10-15 years ago many of the scientific experts were saying that prevention by genetic screening for Hemochromatosis was not possible or sensible. For example, at that stage it was thought that a small percentage of people with the high risk gene mutations would actually be symptomatic for HH - figures like 10% penetration were often given for even the worst Homozygous C282Y mutations were given. Since then much further scientific work has been done and real measurements have now been published. The published figure for Homozygous C282Y biological penetration is 77% and the reason why this is not 100% is simply that some people have other mutations which slows the overload process so much that many die before they become overloaded. There was also an issue which is not admitted in that DNA analysis was very expensive at that stage and certainly medical organisations did not want to feel the pressure of being asked to screen for Hemochromatosis.HH Carriers
There are also often ommissions about HH carriers. It was always known that a very low percentage of carriers suffered from Iron Overload, in fact its now known that only between 1-2% of carriers do suffer from iron overload. The medical profession have picked up on this number and simplified it to the extent that some often say there are "no risks for carriers". In fact statistically, they will be right for 98% of the time they say it and for these people its correct to not worry about it ever again. However, for the 1-2% left this is very incorrect advice. Further more this 1-2% of people is rather a large number of people, because between 8% and 23% of the whole population are indeed carriers. The table below shows the actual numbers for the US

You can see from the above table that there are more H63D carriers (H63D/wt) suffering from Iron Overload than there are Homozygous C282Y people who, we are often told, are the most common suffers. Very few in the Hemochromatosis community state or even know this statistic fact correctly at all.
So even though there is a lot of useful information in the other online HH resources there is also some important ommissions and even incorrect statements because they are presenting information 10-15 years out of date. So here we present the latest scientific informations.
Prevention using Genetic prediction of Age at Symptoms
In the overview section we've introduced the concept that a DNA test can help determine the risks for the user. We also explained that historically this approach was not believed possible and even today most people including most medical experts will say that Prevention using a DNA test is not possible.
Current guidelines (the best of such Guidelines is BHS Diagnosis and therapy of genetic haemochromatosis (review and 2017 update)) still only suggest DNA tests for close relatives of homozygous C282Y sufferers and for others it suggests blood tests for ferritin and transferrin saturation are the main ways to determine risks with later possible support of a DNA test only if iron levels are determined to be raised. It's, of course this way round, because historically DNA tests have been expensive and it is believed to be a cost saver to do the cheaper blood test first. This cost saving is debatable because by waiting for the blood iron levels to be raised enough to indicate Hemochromatosis the patient is exposing him/herself to iron levels which can cause signficant organ damage. This is the basis of the problem we have with this approach - we believe that we can show that a commercial DNA test alone can predict when someone will get symptoms.
Now let's look at the types of Genetic Testing. There are 2 main ways of a patient getting a Hemochromatosis Genetic test. The first is from
your doctor who will do a genetic blood test -often this test is limited to looking just a the mutations of the HFE gene but in some cases it
can be extended to look at many more mutations such as listed in this picture. 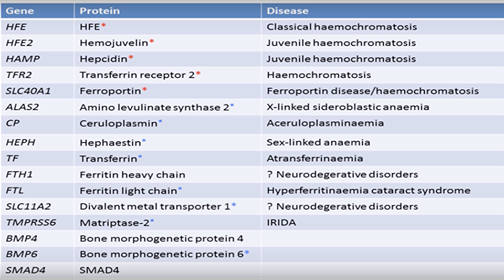 .
.
This list
of 16 genes is more useful but is still missing the important BMP2 gene mutations. This youtube video describes the work being done here and says
this test costs the NHS £500. (see this video for the details of the specialised 16 gene NHS test)
Meanwhie the second way is for you to do a more extensive tests using one of the commercially available tests from the Family Tree type DNA tests (eg Ancestry, 23andMe, MyHeritage, FamilyTreeDNA etc). These tests look at hundreds of thousands of mutations and the result is that because of the extra data a more extensive analysis can be performed. Indeed, it's the use of these tests which has enabled us to spot patterns missed by the very limited testing performed by the medical profession. The cost of these tests can be as low as US$99 and even lower at times of special offers. Often people say these commericial tests are not medical grade and indeed they are not medical grade but it is better to consider accuracy. The accuracy of these medical tests is no different to the accuracy of these commercial tests, but they do not have the label "medical grade". In my experience though my doctor had no problem with my AncestryDNA result - he totally accepted it.
At the time of writing we've had 170 uploads in CheckIron V1 but only have had 21 of those that told us their age when they got symptoms. We had hoped for more, but this has been enough to give us some confidence that there is a pattern between certain mutations and the age at symptoms. In fact we discovered that the age of symptoms for Hemochromatosis Type 1 is dependent on 2 major factors (this is a simplified view):
- The HFE gene mutation - C282Y or H63D in every combination (double, single or combined)
- The transferrin mutations - we found that BMP2 and Pro570Ser in particular mutations speed up loading and the G277S mutation slows loading.
Both these factors are highly significant whereas we believe many others are just using the HFE mutations are the single indicator of Hemochromatosis and as a results carriers and even combined mutations are being ignored as being significant. We believe all the combinations are signficant in the presence of the transferrin mutations which increased transferrin.
So that was the simplified view. When we calculate the Age at Symptoms we also include other factor such as the risk of other auto-immune diseases due to other gene mutations - the effects here are smaller but it is also taken into account. Currently the calculations are raw estimates but over time and with increasing input from users uploading their data we hope to make these estimates more accurate. We do accept that external factors like, diet, and blood loss through menstruation or blood donation also have a non genetic input to the age at symptoms.
Age at Symptoms
Hemochromatosis causes iron to build up in the body over time. So it's ususally at an older age people suffer most. But why is it that for the same genetic mutation there is a wide range ages people begin to suffer (we call this the age at symptoms).
| Homozygous C282Y | Compound C282Y/H63D | Homozygous H63D | H63D Carrier | C282Y Carrier | No HFE Mutations | |
|---|---|---|---|---|---|---|
| Penetrance Chart | 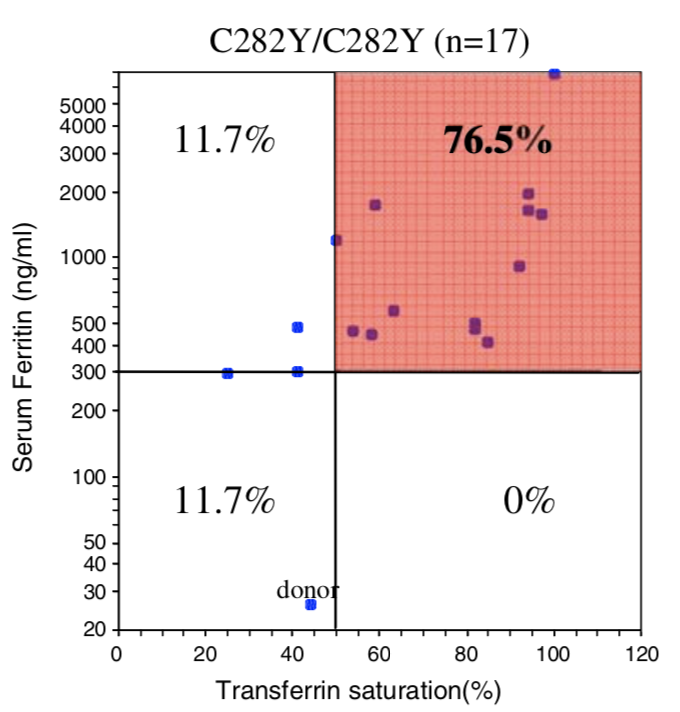 |
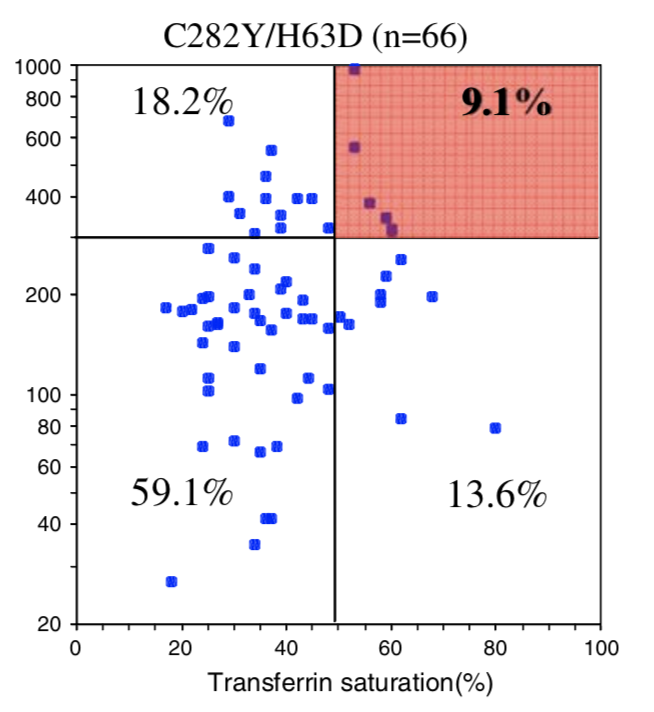 | 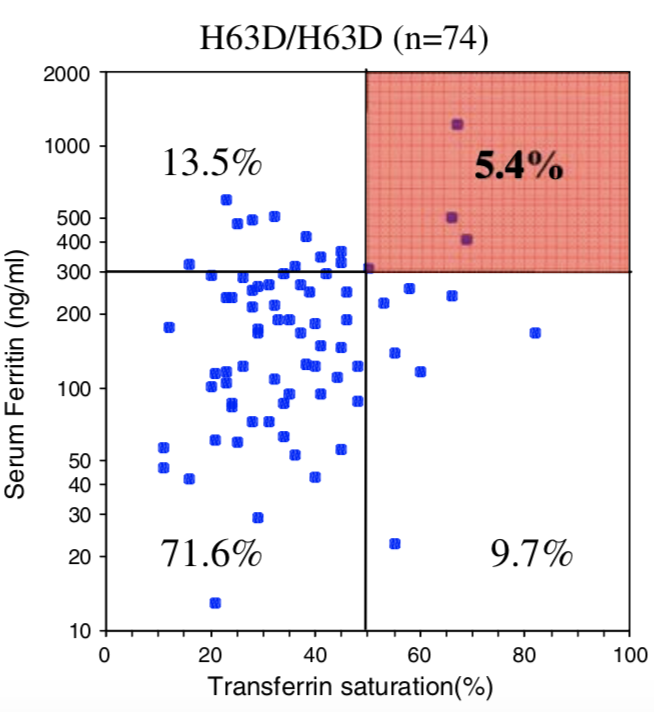 |
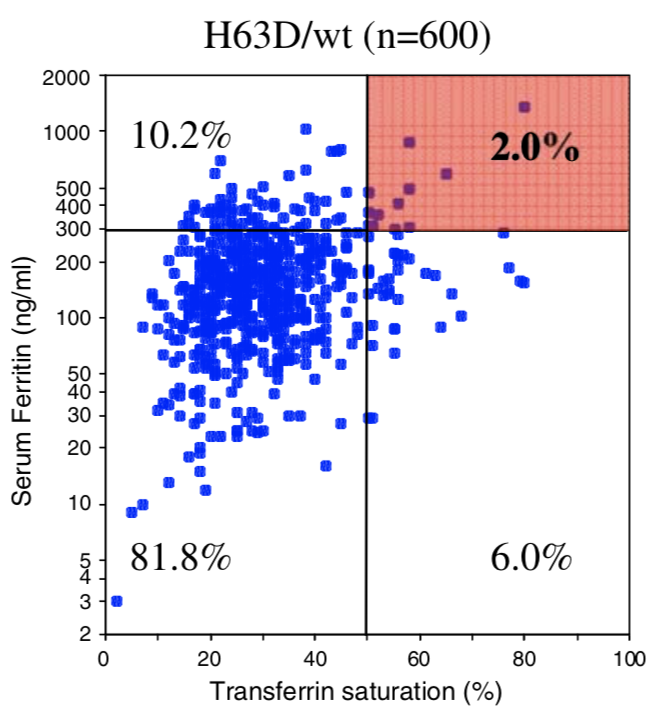 | 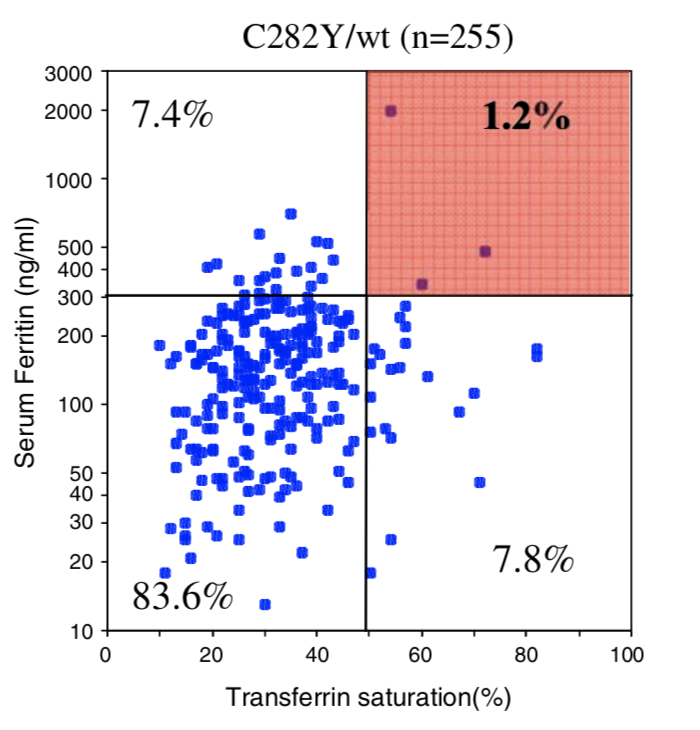 |
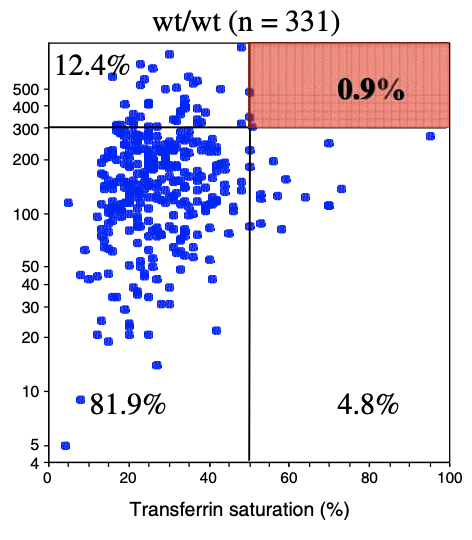 |
| Biological Penetrance | 76.5% | 9.1% | 5.4% | 2.0% | 1.2% | 0.9% |
| Age range for symptoms | 16-100 years | 34-100 years | 41-100 years | 41-100 years | 33-100 years | uncertain |
You can see from the above table that there are massive age range for all the HFE Gene mutations and their combinatons but for the lower penetrance ones the age range is slighly less. The reason for this is that the homozygous C282Y mutations causes the strongest iron loading, whereas the carrier (single mutation) examples have much slower loading rates. But this still does not explain why their is such a wide age at symptoms range for all cases. And the reason for this is there is something else going on. It could be 1 of 2 reasons or both of these:
- Either a lifestyle thing - different people eat different iron containing food or regularly loose blood for example via blood donation
- Or, another mutation is having just as large effect as the HFE gene.
The reality it IS going to be both but it appears based on our data that in reality it is for most people mostly the effect of other mutations
Now let's look at what's going on here.
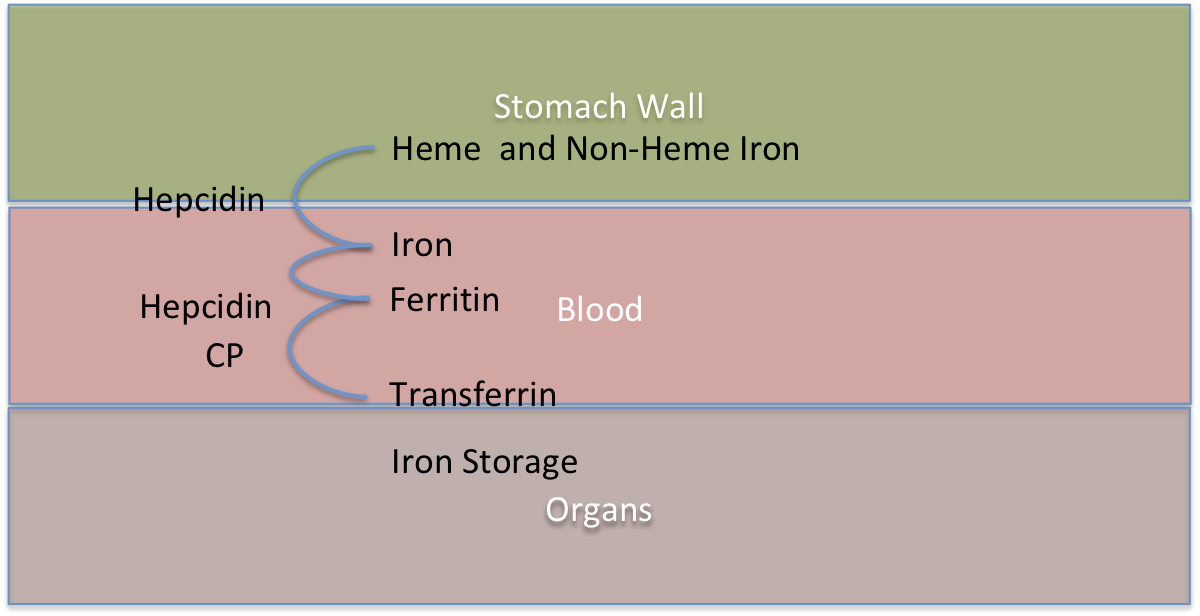
The diagram shows a simplified view of what is happening with iron in the body. There are 3 layers involved, the stomach/duodenum wall, the blood and then the organs. Classic Iron overload is mostly concerned with too much iron in the organs and it accumulatives over time. But serum iron overload (SIO) also exists as a lesser complication with different symptoms. SIO needs to happen before organ iron overload (OIO) can occur. Whereas SIO is dependent on a defect in Hepicidin production for Hemochromatosis Type1, OIO is dependent on Transferrin production to carry the ferritin into the organs and mutations of the Transferrin (TF) change the rate at which this happens. This classic organ iron overload is dependent on 2 genes and defects in both genes either increases or decreases the rate at which the iron overload accumulate. It is therefore the mutations of both HFE and TF genes which determines what age we will overload. Throughout life the iron in our organs accumulates and when it gets to a point where it becomes too much then the damage to the organs starts to occur and we experience the autoimmune symptoms of iron overload.
The important thing to realise is we measure the ferritin and transferrin in the blood to determine if we have iron overload but this is an approximation to measuring the iron stored in the organs. As it is, the excess iron in the organs which causes the majority of the medical issues. The HFE gene mutations controls the rate at which the iron flows from the stomach/duodenum wall into the blood-when a person has one of the Hemochromatosis mutations the bodies is less able to make a protein called Hepcidin. In healthy people Hepicin controls the rate of flow of iron into the blood stream - if the body has enough iron the body can request no more iron comes in - in Hemochromatosis this request does not work as well and the iron continues to flow in.
The above is a simplification and really only describes the way Hemochromatosis Type 1 occurs. Other less common conditions can complicate everything much more and we'll discuss these in a future article.
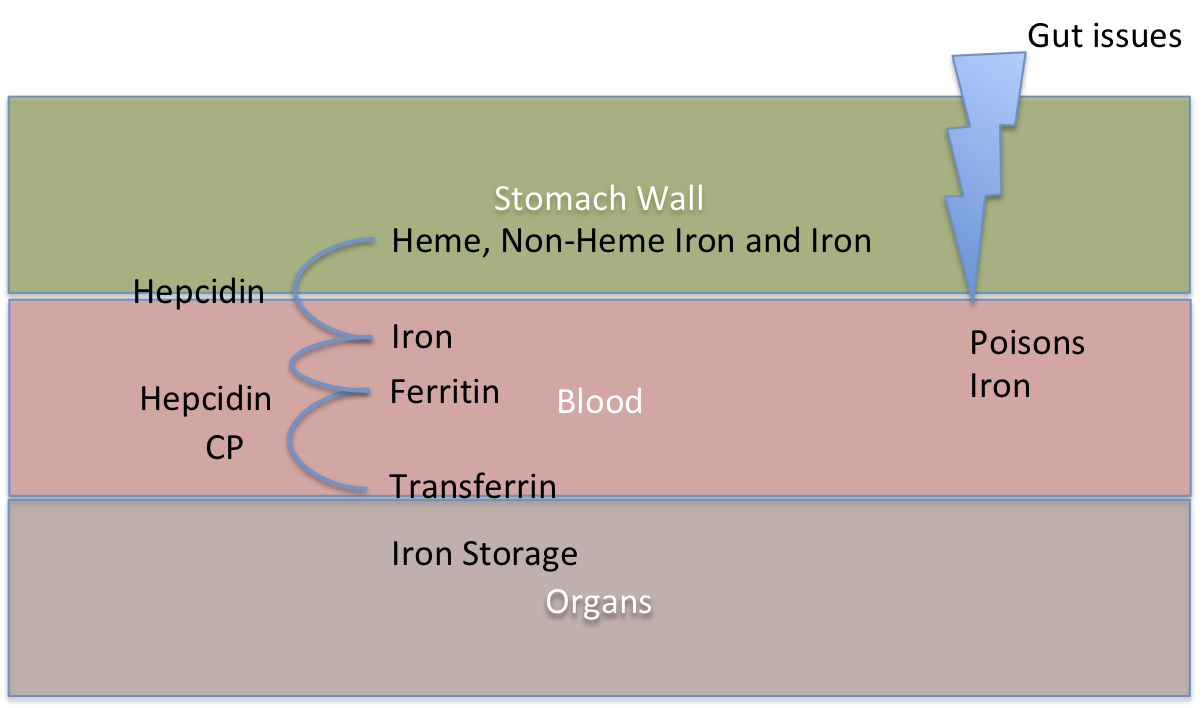
To further complicate matters if we have the mutations to cause Hemochromatosis and we also have Gut issues such as Gluten Intolerance or even an ulcer, there is a more direct means of iron and other poisons getting into the blood. This means those with the mutations will experience unimpeded iron flow into the blood. So it then just takes a TF mutation which increases Transferrin to then cause more iron to flow into the organs. The worst thing a person can do with Gut issues is to eat fortified breakfast cereal which contains pure iron already which can just cross the gut barrier with no problems.
CheckIron is now getting more and more people who upload their data and for those that have been ill, they tell us the age when they got symptoms. This
is very useful for it allows us to perform data analysis on the data to determine what mutations are causing their symptoms. Initially when we started this work
we only looked at the iron metabolism mutations and we immediately began to see a pattern. Below we have a graph of 34 people and we plot for each person we plot their HFE mutations
as 5 separate columns showing age at symptoms on the y-axis
For each user we show the mutation iron overload modifier mutations as a horizontal line of dots.
The redish points are modifiers which speed up loading
of iron and the green modifiers are mutations which slow the loading of iron.
The pattern we saw immediately was that the redish points are low down and the greenish points are higher up.
In other words the speed of loading is really affecting the age of symptoms as predicted by our theory.
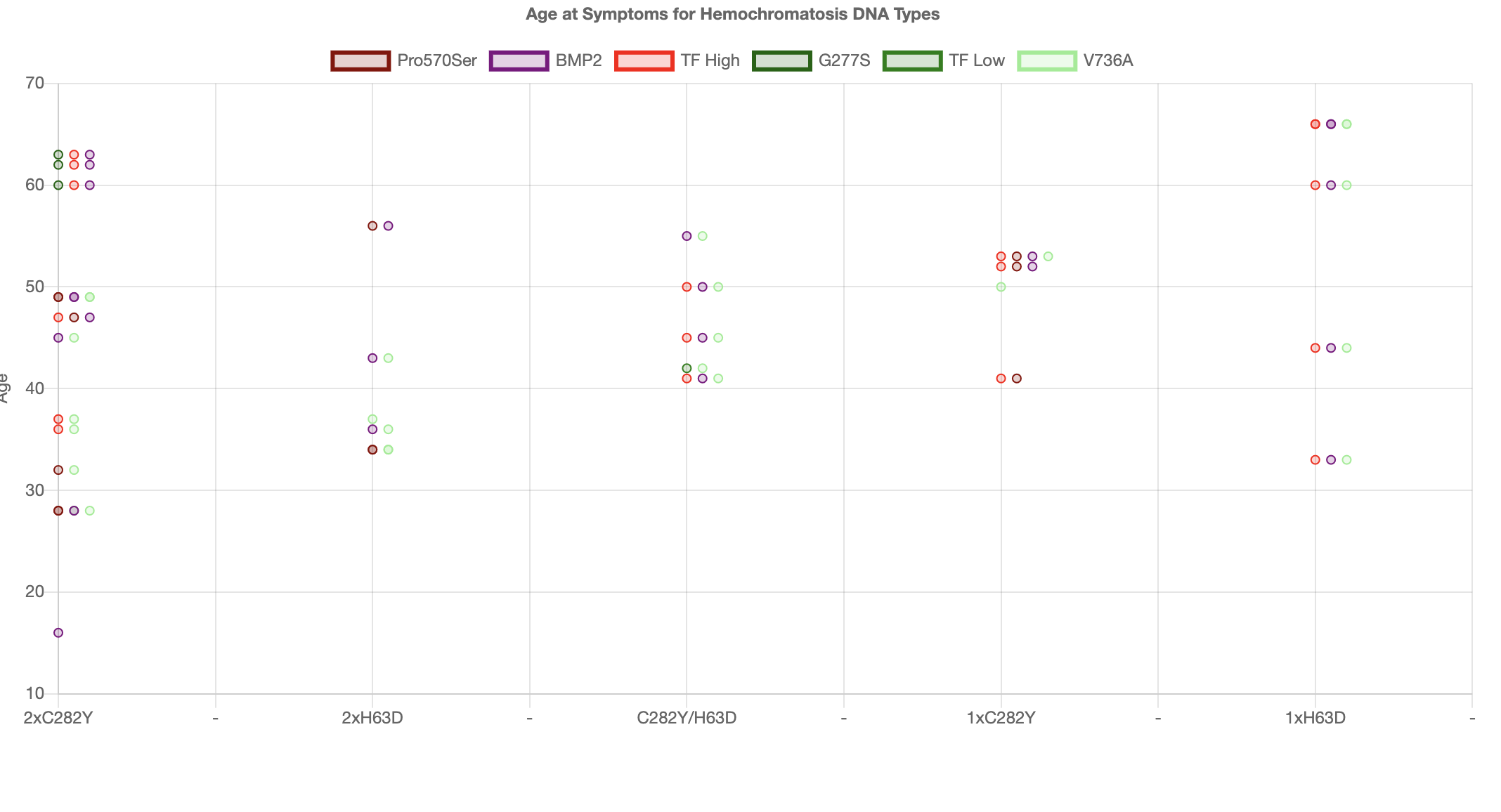
But you can also see that for carriers the effect does not
work so well. One aspect of carriers is that because the hemochromatosis effect is much less other inflammatory conditions are also
contributing to symptoms which are indistinguishable from Hemochromatosis. That is there are several inflammatory conditions possible
that all cause the same inflammatory symptoms.
So below we replot the same graph but include the risks for diseases like Diabetes, Lupus, Hyperthyroidism etc. You can now see that
whereas the iron metabolism modifiers did not work for carriers they start to work for all inflammatory condition risks. We particularly
see a strong effect for Diabetes.
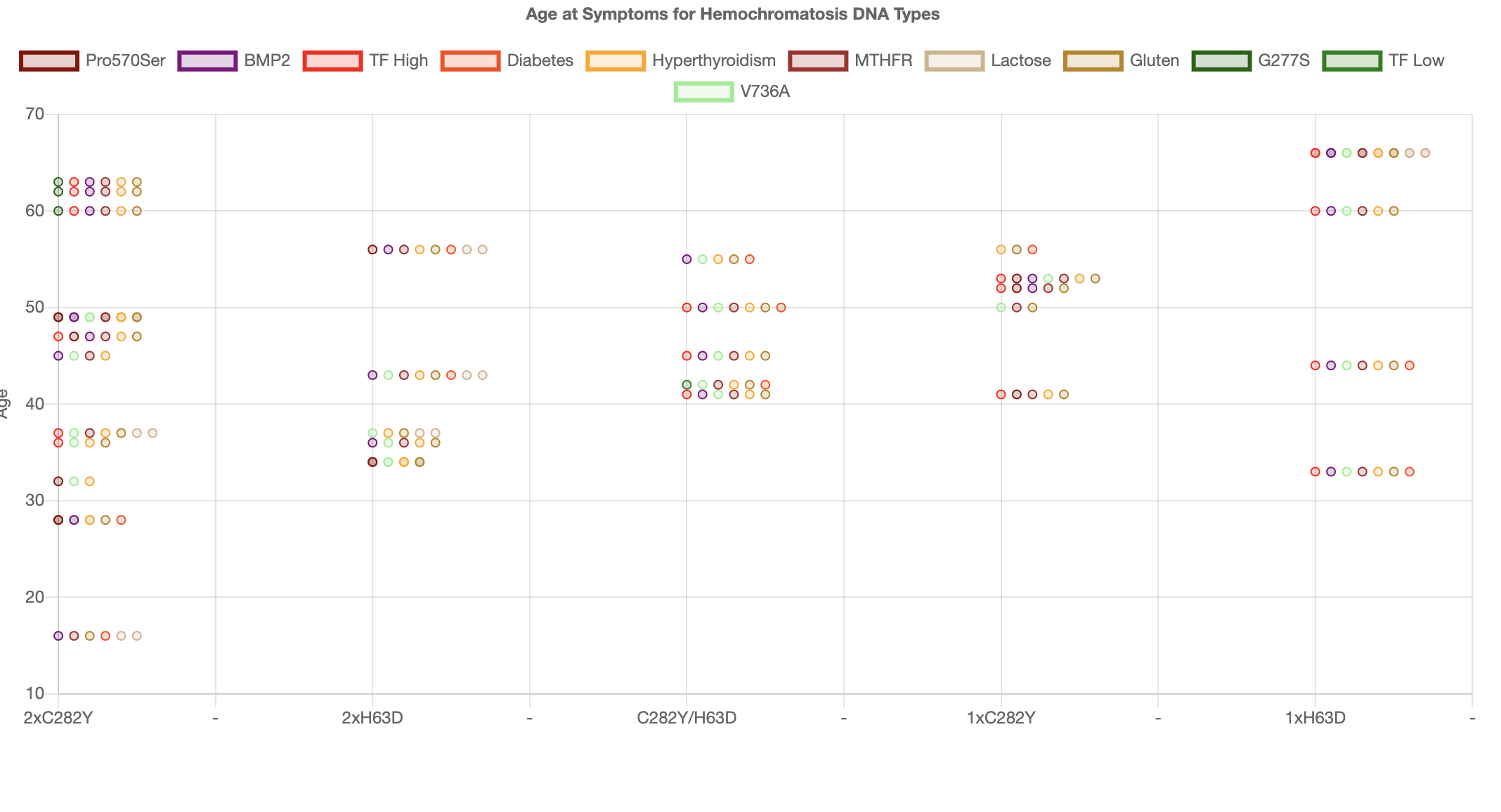
In fact we saw the strong effect from Diabetes risks when we started using Machine Learning to analyse the data. The Machine learning algorithm
immediately predicted risks from Diabetes making up to 16 years lowering of the age at symptoms. Clearly having only 35 results is not enough
data and this is particularly true when we've added many more factors to be fitted to the data. We'll be updating these graphs as the data
grows over the coming months/years.
Worried? Don't Panic
If the DNA analysis has told you, you are at risk of Iron Overload but you are worried because you don't understand what this means, the important thing is not to panic. If you have this condition it is fully treatable so long as not too much damage has been done. If you used CheckIron, we will also have given you an "Age at Symptoms" and for some people this may be worrying, particularly if the age is in the past and you don't yet have symptoms. This age is very much an estimate and it is not yet very accurate -so you don't need to worry. If you are at risk or even if you have any concerns consult your doctor and ask for an iron blood panel test which tests Ferritin and Transferrin Saturation. Note that some doctors will only do the Ferritin test to start with. Also if you are a carrier for Hemochromatosis some doctors will not believe you have any risks although see our prevention article to see what the official risks really are. In our view (although not yet accepted by the experts), we see from the data that carriers have between 1 and 2% chance of overloading iron but this these risks are significantly increased if you have 2 or more mutations of the TF gene which speed up loading. Clearly there are other conditions which speed up loading so you definitely should consult a doctor to get an expert opinion on you personal medical condition - note everyone is different in reality.
If you have the risk mutations of the HFE gene, your doctor should perform an iron panel blood test. Iron levels acuumulate over time, so even if you have a negative result, your doctor should schedule at least yearly tests. If the doctor sees at large Ferritin level (close to or above 1000) plus a Transferrin saturation percent above 45% for a woman or 50% for a man then this would indicate a higher risk of iron overload. In some case doctors consider this enough for a diagnosis but others consider a measurement of the amount of iron in the liver to be needed for confirmation. A form of magnetic resonance imaging (MRI) scan called FerriScan or LiverMultiScan (these are brand names) is now increasingly in use as a way of directly measuring liver iron concentration. MRI is usually preferred to biopsy as it is cheaper, non-invasive, quicker and more accurate. However in some cases a biopsy might still be necessary; a small sample of the liver is removed using a biopsy needle, which is checked to see whether tissue damage such as cirrhosis is present. Liver transient elastography or Fibroscan (a brand name) is similar to an ultrasound scan and is widely used as a way of checking for fibrosis and more severe forms of liver damage such as cirrhosis.
If you have the gene mutations you should consider there are risks for your children, parents, sublings, cousins and aunts and uncles. You should inform your relatives and suggest they also have their DNA checked. This can be done by a doctor or using consumer DNA analysis company and then CheckIron. These 2 types are equally accurate but since the commercial tests, test for many more mutations they are very much better (this latter statement might be considered contraversial by some people).
Once you have a diagnosis then treatment is arranged. The most efficient way to get rid of iron from the body is to do a regular Phlebotomy (called Venesection in the UK) which means taking 1 pint of blood every 1-2 weeks until the ferritin level is decreased. This process will take months or even more than 1 year in some cases. Once the ferritin level is down to 50 then you are deemed to be in maintenance. At this level the Phlebotomies need only doing every 3 months or in some cases 6 months. When is maintenance you are no longer suffering from iron overload and for many countries blood donation is also allowed which can be a useful alternative to Phlebotomies. Note that even when the ferritin level has decreased the Transferrin Saturation is likely to stay high to begin with, but this too will eventually fall. The case of low ferritin and high Transferrin Saturation is sometimes called Iron Avidity (there seem to be some myths put out around about this state, Ignore these stories, they are usually meaningless.)
Inflammation
The Cause and Effect Tree involving Iron(Fe) and Inflammation
Cause and Effect within the Human Body is more often than not confused and misinterpreted by Doctors. It IS complicated because there is often a chain of causes and effects and additionally there can be a feedback loop where the effect links back to the cause.
These 2 complications are particular true for Inflammation. We show a typical Cause and Effect tree in the picture below for Inflammation and furthermore we spell out the Role of pure Iron in the inflammation story which is nearly always ignored as though its not highly signifcant as it really is.
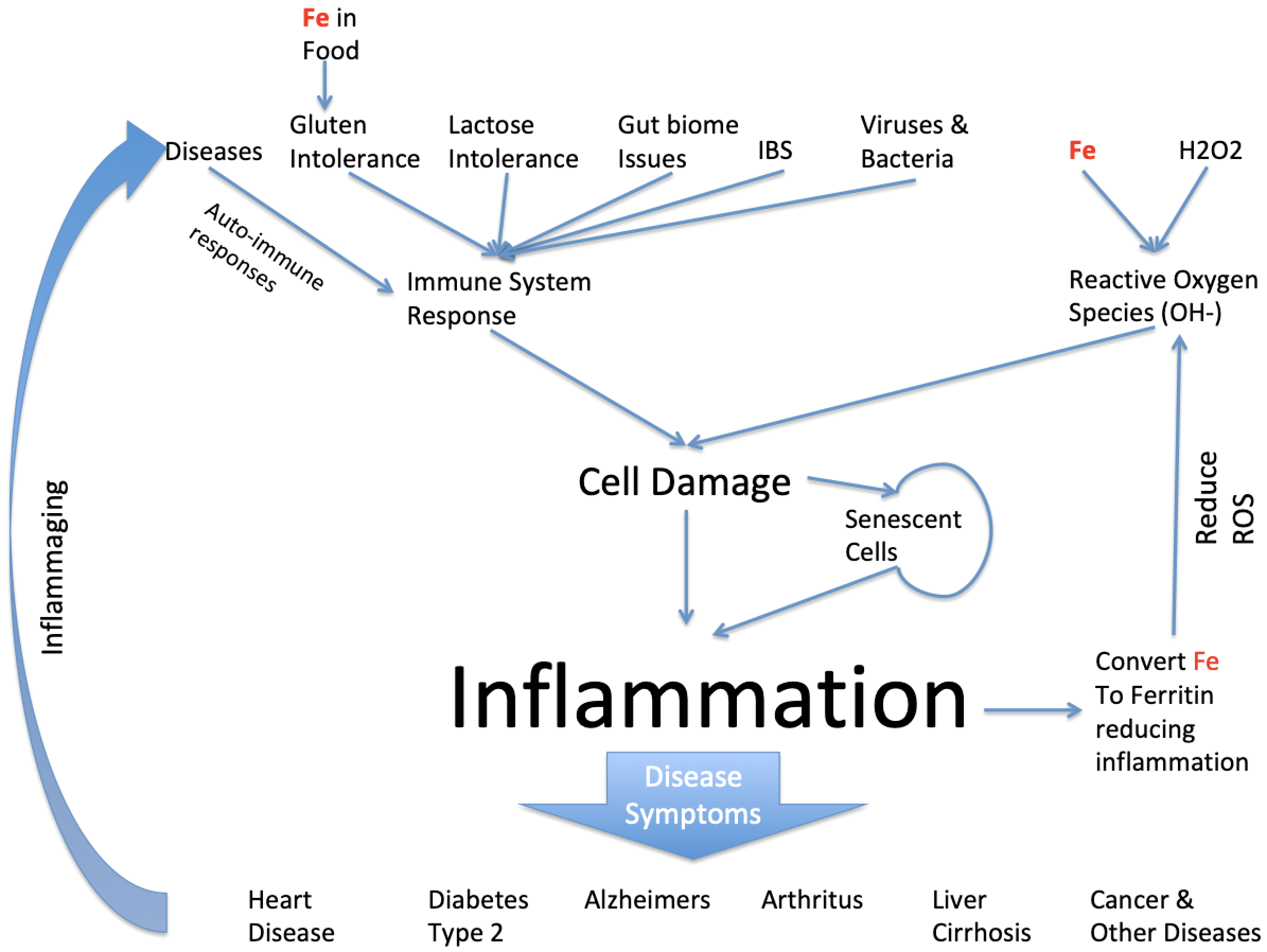
Clearly inflammation can be caused by a large number of things but we're particularly interested in the role iron takes in this and it does so in a number of ways. First iron enters the body through food eaten and usually in a biochemical form. However, when iron is added to foods for alleged nutritional reasons the iron is more often than not added as pure iron. Pure iron is highly toxic in conjunction with Gluten Intolerance it can enter blood stream directly and cause havoc by inducing inflammation from creation of Reactve Oxygen Species (ROS). The pure iron reacts with natural small amounts of natural Hydrogen Peroxide in the body resulting in Hydroxyl ions which are highly damaging to cells it comes across and cause inflammation. The body always has some naturally pure iron because the immune system of a healthy person can control how much there is. But if the person has Hemochromatosis this is not the case.
Meanwhile there are many other factors involved in the creation of inflammation. Its main exisitence is due to the immune system attacking foreign invaders such as Viruses and Bacteria. The side effect of it is that it can also attack close by healthy tissue. The immune system will also attack milk products if the person cannot process lactose and this also causes inflammation. Once a cell is damaged it can effectively stop working but its left in a senescent state. Senescent cells can infect close by cells making them senescent too and causing further inflammation. There are mechanisms to remove senescent cells but I'll address that in a separate article.
Dependent on where in the body the inflammation occurs results in the disease we observe, so in the heart its called heart diseases, in the brain its called Alzheimers or Parkinsons and as a result of being in the pancreas with observe diabetes because the person has insulin resistance and cannot control their blood sugar levels.
And my point was that iron is very significant in this story both as either excess iron because its been build up in the body because of old age and for the 1.25% of the population with the most common genetic disease of Hemochromatosis its highly significant as it can be a significant contributor to all or some of these inflammatory diseases. Another point is that the other root causes such as Diabetes, Gluten and Lactose intolerance are also genetic risks.
Since all these diseases are mostly age dependent and which ones we suffer from are dependent on our genes, I want to ask the question can we predict, given knowledge of our DNA, what age you will suffer from one or some of these diseases. Clearly lifestyle (ie what we eat) comes into this too but assuming most people have similar lifestyles can we infer from genes what age someone will start suffering from these Inflammatory diseases. Now I run a website (called Checkiron.com) which helps people that suffer from Hemochromatosis. Its been running 12 months and I’ve been collecting their DNA data and asking them when they got symptoms- we then use Machine Learning techniques to learn and then infer from a new DNA dataset when someone will get symptoms. To start with I was just looking at the genes involved in the Hemochromatosis disease and this worked for the extreme cases when people got inflammatory diseases in their 20s but it worked less well for the weaker forms of Hemochromatosis and we discovered from the data it was things like Diabetes Type 2 disease that were having a significant affect on the “age at symptoms”. Simply because all these symptoms of inflammatory diseases are indistinguishable for the patient and the doctor treating the patient. The result is that currently for people with the Hemochromatosis mutations we can predict to +-8 years when someone will get bad symptoms. Furthermore since Hemochromatosis is entirely treatable, if caught early enough, we can say at what age, this preventative treatment should be taken. This approach could save 100s thousands of lives each year of Americans suffering from HH and not knowing it. Furthermore since this disease runs in families then those that detect the issue could suggest to other family members they should check.
FAQ
FAQs
None of the below should be considered medical advice - we are describing the questions people ask about Hemochromatosis. Always consult a qualified medical professional.
- Official Recommended Procedures is to do Iron Panel Tests before Genetic tests. Why are you saying something different?. These procedures were created at a time when it was not known that DNA could fully predict the "age at symptoms". Since this is now known those procedures are now invalid.
- Only 10-20% of people with the HFE mutation get Hemochromatosis. So what is the point in doing a DNA test?. This 10-20% number is for clinical penetrance which happens when the organs are damaged. Now we know someone has HH when they reach Biological Penetrance clinical penetrance is now a meaningless condition since more and more people are being treated at Biological Penetrance.
- It does not matter what I eat for iron and HH. Do you agree? This is a simplification which has been blown out proportion by being repeated too many times by people who don't understand what's is purpose is. If you as a HH sufferer with iron overload are under-going phlebotomies then it does not matter what you eat within reason because your condition is being treated by the much stronger effect of Phlebotomy. However, before Biological Penetrance is reached or after maintenance is attained it is better for the body NOT to be subject to the inflammatory effects of too much iron.
Sample Data
You may be interested in seeing example data for different people with different mutations. Why do different people with the same HFE mutations overload any where between age 20 and age 70? The answer is the other mutations are affecting the iron loading rate. See deifferent people with different loading rates affects their age at symptoms figures. These datasets are based on real people but have been altered slightly so there is no connection to a particular real person.
The examples are as follows:
- Homozygous C282Y with fast loading
- Homozygous C282Y with slow loading
- Carrier H63D with fast loading
- Compound C282Y/H63D fast loading
- Compound C282Y/H63D slow loading